Company Introduce
Hochuen Medical is a leading OEM manufacturer for disposables & devices in the medical (IVD and POCT), life science, drug delivery, veterinary diagnostics, surgery, and medical wearable spaces. We provide turnkey services for customers and provide support from the R&D and prototyping phases to low and high-volume CDMO manufacturing on microfluidic chips and biomedical consumables. Hochuen Medical is FDA registered and ISO 13485 certified. Our manufacturing sites consist of class 10K-100K cleanrooms and a GMP facility. Hochuen Medical is based in Los Angeles, CA, USA and has an office in Singapore and manufacturing sites in Malaysia and China (Shenzhen and Dongguan).
Exhibit brands
Exhibits
Laser Welding
Laser Welding Description: Laser welding is suitable for seal joints without adhesives. It achieves partial or complete bonding through laser melting and can be performed with a vision system for precise alignment and welding.
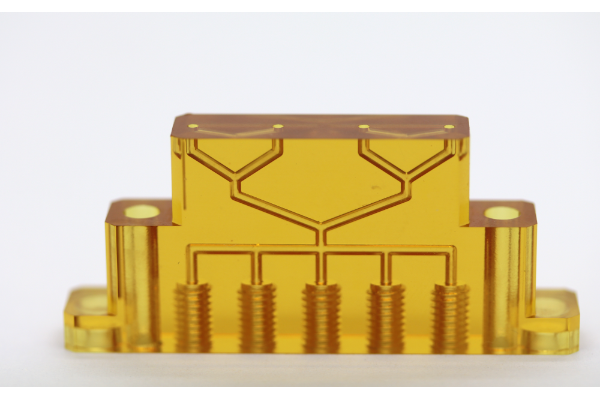
Flow Divider
Description: The Flow Divider is widely used in IVD testing instruments and can be customized. It features excellent thermodynamic and chemical properties.
Thermal Compression Bonding
Thermal Compression Bonding Description: Thermal compression bonding is a process that does not require an intermediate medium. The materials on the top and bottom of the chip need to be consistent. It provides high bonding strength and is suitable for most thermoplastic polymers.
Precision Injection Molding
Precision Injection Molding Description: Injection molding is a manufacturing process that uses plastic molds to produce products. This method ensures high consistency and efficiency in product quality.
Chip Assembly
Chip Assembly Description: Chip assembly provides system integration solutions for the assembly of customers' partial products or entire product sets.
Main products
Shenzhen Hochuen Medical Technology Co., Ltd. is a national high-tech enterprise focusing on in vitro diagnosis, life science and surgical instrument OEM business, especially in real-time diagnosis, microfluidic devices and non-standard customized products to reach the industry-leading level.