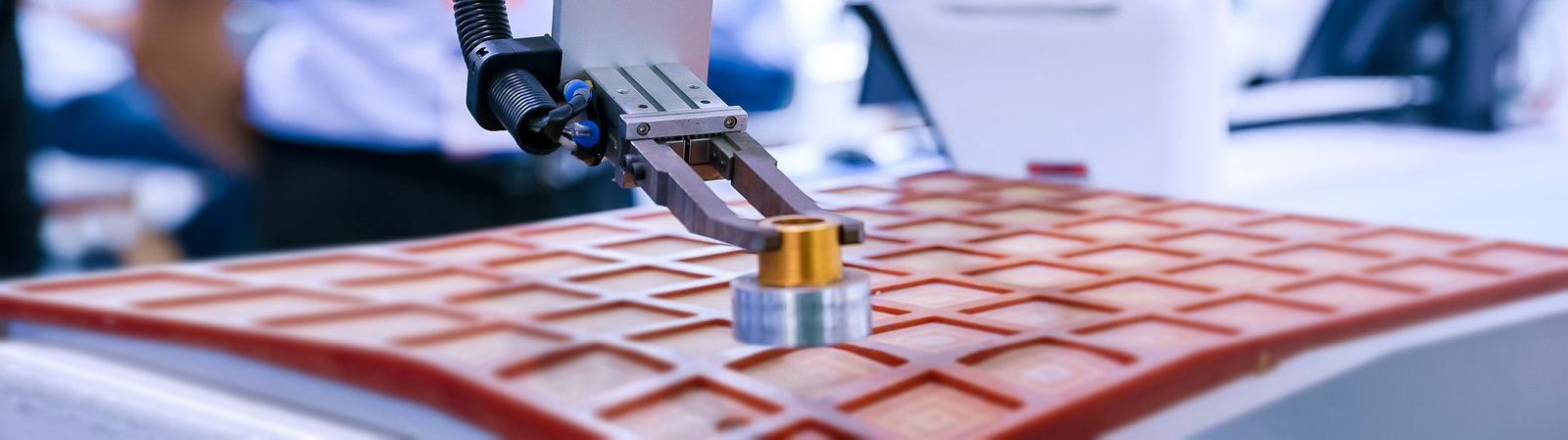
Injection Molding Automation for High-Volume Medical Consumables
【Introduction】 Injection Molding Automation for High-Volume Medical Consumables
Producing medical consumables—from specimen collection tubes to surgical stapler cartridges—operates on a different paradigm than standard manufacturing. The imperative for sterility, lot traceability, and absolute consistency across millions of parts necessitates a manufacturing approach built on control and repeatability. Within this field, the progression from manual molding to fully automated production cells represents a significant operational evolution. We at ITES China observe that automation within China medical injection molding is not merely about labor savings; it is a foundational requirement for achieving the quality and scale demanded by global healthcare markets. This integration of robotics, integrated quality checks, and data tracking transforms a molding machine into a validated production system.
.png)
Core Components of an Automated Molding Cell
A functional automated cell for medical injection molding services extends far beyond the press itself. It typically integrates several key subsystems. Robotic arms, often cleanroom-compatible, perform the consistent demolding and placement of parts. Conveyance systems, such as belt conveyors or linked buffer stations, move components to secondary operations. Crucially, in-mold or in-line vision inspection systems verify critical part dimensions and detect defects like flashes or shorts in real-time, enabling immediate rejection. These elements are orchestrated by a central controller that synchronizes the machine cycle with peripheral actions and gathers production data. This closed-loop environment minimizes human intervention in the molding area, directly reducing contamination risks and variation, which is essential for items like IV connector bodies or inhaler components.
The Integrated Supply Chain for Medical Molding Solutions
Implementing such a system efficiently relies on a specialized and responsive manufacturing network. The development of advanced China medical injection molding capabilities is supported by a clustered ecosystem. This includes direct access to makers of servo-electric and hybrid molding machines designed for cleanroom use, specialized tool steel providers, and manufacturers of auxiliary equipment like dryers, chillers, and granulators that meet medical cleanliness protocols. For companies working with ITES China, this proximity allows for closer collaboration between mold designers, automation integrators, and material suppliers. A molder can rapidly prototype a new consumable, source medical-grade resins from a nearby compounder, and work with an integrator to design a turnkey cell with validated automation, significantly compressing the time from design to certified production.
Addressing Industry Challenges at ITES China
The upcoming exhibition features a zone specifically dedicated to solving the pressing challenges in this sector. The Injection Molding Technology Supply Chain Exhibition Zone is structured to address known industry pain points, including equipment interoperability, insufficient molding precision, and misalignment between mold design and production outcomes. This focus provides a targeted resource for industry buyers. The zone presents a comprehensive view of the core technical chain, bringing together specialists in three interconnected areas: advanced plastic mold design and manufacturing for complex medical parts, precision injection molding equipment paired with tailored automation technologies, and the crucial selection of biocompatible and high-performance injection molding raw materials. This collective display is engineered to assist visitors in sourcing both advanced technologies and qualified suppliers.
Exhibition Scope: From Mold Design to Validated Production
The scope of exhibits within this zone is designed to guide a professional through the entire technical journey. Visitors can engage with engineering firms that specialize in medical-grade mold design, focusing on features that enhance manufacturability and part quality. Equipment manufacturers will showcase presses with the precise shot control and repeatability necessary for micro-molding or multi-material applications. Critically, automation partners will demonstrate integrated systems for part handling, decoration, and packaging. Furthermore, material science companies will present grades of polymers, such as cyclic olefin copolymers (COC) or specialized polycarbonates, that meet USP Class VI or ISO 10993 standards. This convergence allows for side-by-side evaluation of how mold design, machine performance, automation, and material selection interact to determine the success of a medical injection molding services project.
The transition to automated injection molding for medical consumables is a strategic response to the non-negotiable demands of the healthcare industry for quality, safety, and scale. This approach relies on the seamless integration of precision machinery, sterile automation, and controlled materials. The concentrated supply chain available streamlines the development and deployment of these complex production systems. Our curation of this specialized exhibition zone at ITES China is intended to directly connect medical device brands and contract manufacturers with the complete spectrum of technology providers. It offers a platform to assess integrated solutions, discuss specific application challenges with experts, and identify partners capable of delivering the validated, high-volume production that modern China medical injection molding requires. This gathering represents a direct channel to the expertise and equipment that can define a project's compliance and commercial success.